Exploration of Biomimetic Metal-Organic Catalysts for Splitting Glucose into Dihydroxyacetone and Glyceraldehyde
Downloads
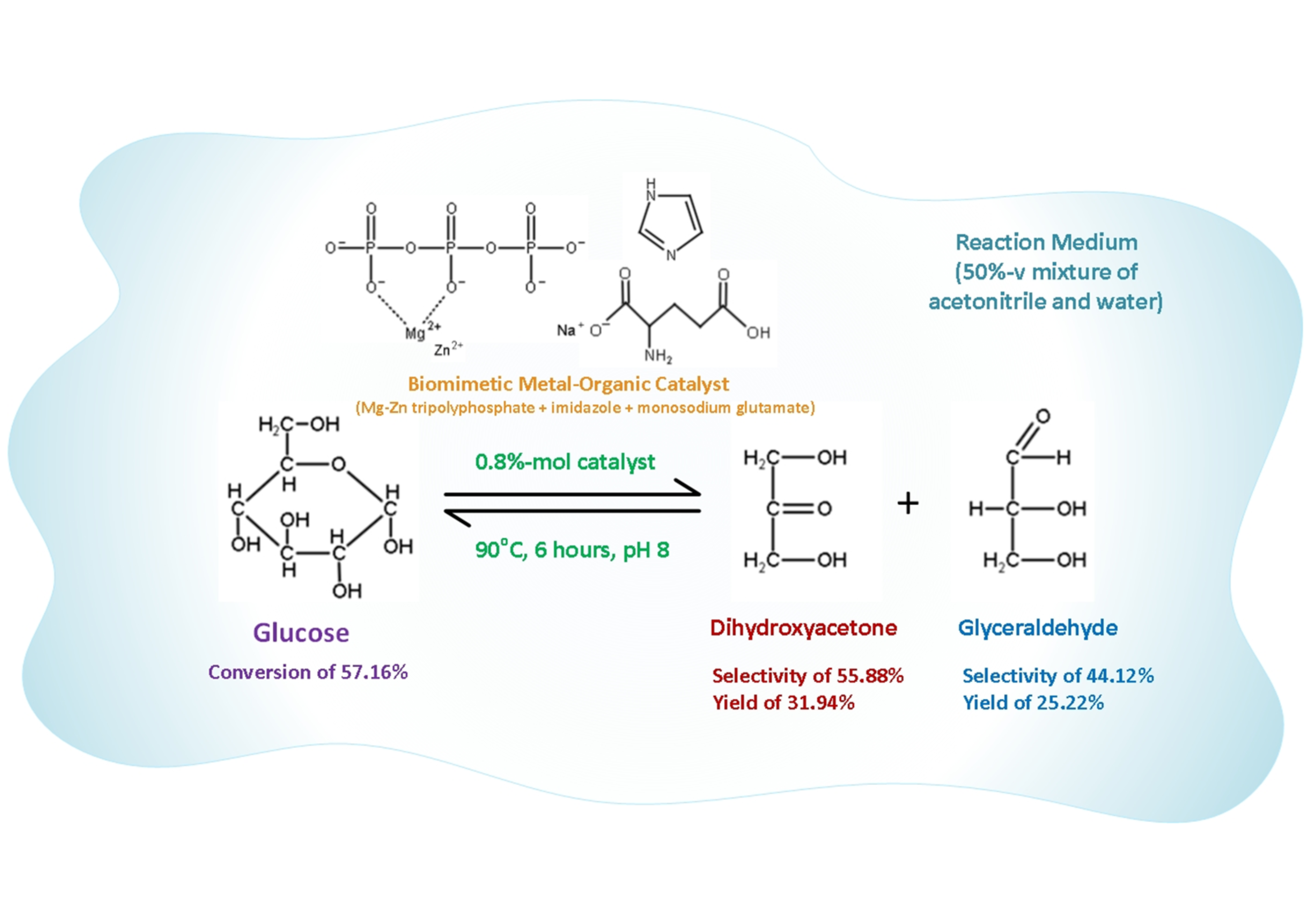
Lactic acid is a metabolite produced during glucose glycolysis, typically generated industrially through the fermentation of glucose using microorganisms. However, the high production cost of this fermentation process makes lactic acid relatively expensive, rendering biodegradable plastics made from polylactic acid less competitive. This research focuses on the initial stages of glucose glycolysis process, in which glucose is split into dihydroxyacetone and glyceraldehyde, both of which are isomers of lactic acid. Biomimetic metal-organic catalysts composed of Mg-Zn tripolyphosphate, imidazole, and monosodium or monoammonium glutamate were tested in a water–acetonitrile reaction medium. Prior to catalyst testing, either water–acetonitrile or water–acetonitrile–acetone solutions were chosen as the reaction medium based on their effectiveness in producing phase separation. The study investigated the effects of catalyst concentrations, catalyst types, and reaction temperatures on glucose conversion, as well as the yield and selectivity of dihydroxyacetone and glyceraldehyde within 6 hours reaction time at pH 8. The results showed that these biomimetic metal-organic catalysts effectively facilitated the splitting of glucose in a water–acetonitrile solution, achieving the best glucose conversion of 57.16% at a temperature of 90°C with a catalyst concentration of 0.8%-mol.
Abedi, E., & Hashemi, S. M. B. (2020). Lactic acid production – Producing microorganisms and substrates sources-state of art. Heliyon, 6(10), 1–32. https://doi.org/10.1016/j.heliyon.2020.e04974
Akoetey, W. (2015). Direct fermentation of sweet potato starch into lactic acid by Lactobacillus amylovorus: The prospect of an adaptation process (Graduate Theses and Dissertations). University of Arkansas, Fayetteville, United States.
Arcos, J. A., Bernabé, M., & Otero, C. (1998). Different strategies for selective monoacylation of hexoaldoses in acetone. Journal of Surfactants and Detergents, 1(3), 345–352. https://doi.org/10.1007/s11743-998-0035-1
Asnis, R. E., & Brodie, A. F. (1953). A glycerol dehydrogenase from Escherichia coli. The Journal of Biological Chemistry, 203(1), 153–159. https://doi.org/10.1016/S0021-9258(19)52625-4
Balogh, S., Kolos, E., Gall, G., & Kolonits, P. (1975). Beiträge zur titrimetrischen Bestimmung von dimeren Hydroxyaldehyden. Zeitschrift für Analytische Chemie, 276(3), 201–204. https://doi.org/10.1007/BF00437567
Bhosale, S. H., Rao, M. B., & Deshpande, V. V. (1996). Molecular and industrial aspects of glucose isomerase. Microbiological Reviews, 60(2), 280–300. https://doi.org/10.1128/mr.60.2.280-300.1996
Blacklow, S. C., Raines, R. T., Lim, W. A., Zamore, P. D., & Knowles, J. R. (1988). Triosephosphate isomerase catalysis is diffusion controlled. Biochemistry, 27(4), 1158–1165. https://doi.org/10.1021/bi00404a013
Blanco, G., & Blanco, A. (2017). Medical biochemistry. In Carbohydrate metabolism (Chapter 14). Academic Press – Elsevier.
Bobtelsky, M., & Kertes, S. (1954). The polyphosphates of calcium, strontium, barium, and magnesium: Their complex character, composition, and behaviour. Journal of Applied Chemistry, 4(8), 419–429. https://doi.org/10.1002/jctb.5010040807
Bobtelsky, M., & Kertes, S. (1955). The polyphosphates of cadmium, zinc, and lead: The character, composition, and behaviour of their complexes. Journal of Applied Chemistry, 5(3), 125–133. https://doi.org/10.1002/jctb.5010050305
Bommarius, A. S., & Riebel, B. R. (2004). Biocatalysis: Fundamentals and applications. Wiley-VCH.
Brito-Arias, M. (2020). Enzymes involved in glycolysis, fatty acid and amino acid biosynthesis: Active site mechanisms and inhibition (1st ed.). Bentham Science Publishers.
Cardoso, G. d. B., Mourão, T., Pereira, F. M., Freire, M. G., Fricks, A. T., Soares, C. M. F., & Lima, Á. S. (2013). Aqueous two-phase systems based on acetonitrile and carbohydrates and their application to the extraction of vanillin. Separation and Purification Technology, 104, 106–113. https://doi.org/10.1016/j.seppur.2012.11.001
Chemanalyst. (2023). Lactic acid price trend and forecast. Retrieved December 20, 2023, from https://www.chemanalyst.com/Pricing-data/lactic-acid-1435
D’Amore, T., Russell, I., & Stewart, G. G. (1989). Sugar utilization by yeast during fermentation. Journal of Industrial Microbiology, 4(4), 315–323. https://doi.org/10.1007/BF01577355
DeMan, J. M., Finley, J. W., Hurst, W. J., & Lee, C. Y. (2018). Principles of food chemistry (4th ed.). Springer International Publishing.
Dhamole, P. B., Mahajan, P., & Feng, H. (2010). Phase separation conditions for sugaring-out in acetonitrile-water systems. Journal of Chemical & Engineering Data, 55(9), 3803–3806. https://doi.org/10.1021/je1003115
Feliczak-Guzik, A., Sprynskyy, M., Nowak, I., & Buszewski, B. (2018). Catalytic isomerization of dihydroxyacetone to lactic acid and alkyl lactates over hierarchical zeolites containing tin. Catalysts, 8(1), 1–12. https://doi.org/10.3390/catal8010031
Fukushima, K., Abbate, C., Tabuani, D., Gennari, M., & Camino, G. (2009). Biodegradation of poly(lactic acid) and its nanocomposites. Polymer Degradation and Stability, 94(9), 1646–1655. https://doi.org/10.1016/j.polymdegradstab.2009.07.001
Guppy, M., Attwood, P. V., Hansen, I. A., Sabaratnam, R., Frisina, J., & Whisson, M. E. (1992). pH, temperature, and lactate production in human red blood cells: Implications for blood storage and glycolytic control. Vox Sanguinis, 62(2), 70–75. https://doi.org/10.1111/j.1423-0410.1992.tb01173.x
Handoko, D. S. P., Triyono, Narsito, & Wahyuningsih, T. D. (2009). Effect of temperature on the performance of Ni/Zeolite catalysts in catalytic hydrogenation reactions. Reaktor, 12(4), 218–225. https://doi.org/10.14710/reaktor.12.4.218-225
Hossain, M. A., Mills, K. N., Molley, A. M., Rahaman, M. S., Tulaphol, S., Lalvani, S. B., Dong, J., Sunkara, M. K., & Sathitsuksanoh, N. (2021). Catalytic isomerization of dihydroxyacetone to lactic acid by heat-treated zeolites. Applied Catalysis A: General, 611, 1–6. https://doi.org/10.1016/j.apcata.2020.11797
Imbault, A. L., Gong, J., & Farnood, R. (2020). Photocatalytic production of dihydroxyacetone from glycerol on TiO₂ in acetonitrile. RSC Advances, 10(9), 4956–4968. https://doi.org/10.1039/c9ra09434b
Jain, V. K., Tear, C. J. Y., & Lim, C. Y. (2016). Dihydroxyacetone production in an engineered Escherichia coli through expression of Corynebacterium glutamicum dihydroxyacetone phosphate dephosphorylase. Enzyme and Microbial Technology, 86, 39–44. https://doi.org/10.1016/j.enzmictec.2016.01.015
Jankowski, M. D., Henry, C. S., Broadbelt, L. J., & Hatzimanikatis, V. (2008). Group contribution method for thermodynamic analysis of complex metabolic networks. Biophysical Journal, 95(3), 1487–1499. https://doi.org/10.1529/biophysj.107.124784
Jing, L., Meng, X. G., Huang, H., Wang, F., Yu, W. W., & Wu, Y. Y. (2020). Catalytic conversion of fructose to 1,3-dihydroxyacetone under mild conditions. Catalysis Communications, 145, 106098. https://doi.org/10.1016/j.catcom.2020.106098
Jin, L.-E., Chang, F., Wang, X., & Cao, Q. (2013). Optimization of synthesizing glucose 1-phosphate by sodium tripolyphosphate as a phosphorus acylating agent using response surface methodology. Turkish Journal of Chemistry, 37(5), 765–774. https://doi.org/10.3906/kim-1210-15
Jojima, T., Igari, T., Gunji, W., Suda, M., Inui, M., & Yukawa, H. (2012). Identification of a HAD superfamily phosphatase, HdpA, involved in 1,3-dihydroxyacetone production during sugar catabolism in Corynebacterium glutamicum. FEBS Letters, 586(22), 4228–4232. https://doi.org/10.1016/j.febslet.2012.10.028
Jolimaitre, E., Delcroix, D., Essayem, N., Pinel, C., & Besson, M. (2018). Dihydroxyacetone conversion into lactic acid in an aqueous medium in the presence of metal salts: Influence of the ionic thermodynamic equilibrium on the reaction performance. Catalysis Science & Technology, 8, 1349–1356. https://doi.org/10.1039/C7CY02385E
Kline, G. M., & Acree, S. F. (1930a). A study of the method for titrating aldose sugars with standard iodine and alkali. Bureau of Standards Journal of Research, 5(6), 1063–1084.
Kline, G. M., & Acree, S. F. (1930b). Estimation of aldose sugars by titrating with standard iodine and alkali. Industrial & Engineering Chemistry Analytical Edition, 2(4), 413–415.
McKee, J. R., & McKee, T. (2019). Biochemistry: The molecular basis of life (6th ed., Chapter 8). Oxford University Press.
Parvin, R., & Kalant, N. (1973). Stimulation of glycolysis by imidazole. Life Sciences, 13(9), 1347–1352. https://doi.org/10.1016/0024-3205(73)90155-0
Raisii, A., & Aroujalian, A. (2010). Effects of influence parameters on color formation in glucose syrups during storage. MISC, 42(1), 57–61. https://doi.org/10.22060/MISCJ.2010.196
Sechi, N. da S. M., & Marques, P. T. (2017). Preparation and physicochemical, structural, and morphological characterization of phosphorylated starch. Materials Research, 20(1), 174–180. https://doi.org/10.1590/1980-5373-MR-2016-1008
Setyaningsih, L. W. N., Rizkiyaningrum, U. M., & Andi, R. (2017). Effect of catalyst concentration and catalyst reusability on triacetin synthesis using Lewatite catalyst. Teknoin, 23(1), 56–62. https://doi.org/10.20885/teknoin.vol23.iss1.art7
Sin, L. T., & Tueen, B. S. (2019). Polylactic acid: A practical guide for the processing, manufacturing, and applications of PLA (2nd ed.). Elsevier.
Solomons, J. T. G., Zimmerly, E. M., Burns, S., Krishnamurthy, N., Swan, M. K., Krings, S., Muirhead, H., Chirgwin, J., & Davies, C. (2004). The crystal structure of mouse phosphoglucose isomerase at 1.6 Å resolution and its complex with glucose 6-phosphate reveals the catalytic mechanism of sugar ring opening. Journal of Molecular Biology, 342(3), 847–860. https://doi.org/10.1016/j.jmb.2004.07.085
Sudarmo, U. (2015). Chemistry for SMA/MA XII class. Erlangga.
Sugih, A. K., Loanda, J., & Prasetyo, S. (2019). Synthesis of phosphorylated sugar palm (Aren) starch using low level sodium tripolyphosphate (STPP). Jurnal Bahan Alam Terbarukan, 8(1), 28–33. https://doi.org/10.15294/jbat.v8i1.17685
Sun, S., Wang, Y., Shu, L., Lu, X., Wang, Q., Zhu, C., Shi, J., Lye, G. J., Baganz, F., & Hao, J. (2021). Redirection of the central metabolism of Klebsiella pneumoniae towards dihydroxyacetone production. Microbial Cell Factories, 20, 1–16. https://doi.org/10.1186/s12934-021-01608-0
Thermo Fisher Scientific Chemicals, Inc. (2024). Phosphate, 0.2 M buffer solution, pH 8.0: Safety data sheet. https://www.fishersci.com/store/msds?partNumber=AAJ60480&productDescription=keyword&vendorId=VN00024248&countryCode=US&language=en (1 April 2024)
Vilonen, K. M., Vuolanto, A., Jokela, J., Leisola, M. S. A., & Krause, A. O. I. (2004). Enhanced glucose to fructose conversion in acetone with xylose isomerase stabilized by crystallization and cross-linking. Biotechnology Progress, 20(5), 1555–1560. https://doi.org/10.1021/bp049927j
Wang, B., Feng, H., Ezeji, T., & Blaschek, H. (2008). Sugaring-out separation of acetonitrile from its aqueous solution. Chemical Engineering & Technology, 31(12), 1869–1874. https://doi.org/10.1002/ceat.200800003
Xie, S., Zhang, S., Qiu, X., Yi, C., Hu, Y., Li, F., & Quan, J. (2015). Sugaring-out effects of sucrose and glucose on the liquid–liquid equilibria for the (water + acetone + 1-butanol + ethanol) system. Journal of Chemical & Engineering Data, 60(9), 2434–2441. https://doi.org/10.1021/acs.jced.5b0030
Copyright (c) 2025 Journal of Engineering and Technological Sciences

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.