Deeper Insight into the Rational Design and Synthesis of Zeolites Revealed by Machine Learning: A Mini Review
Downloads
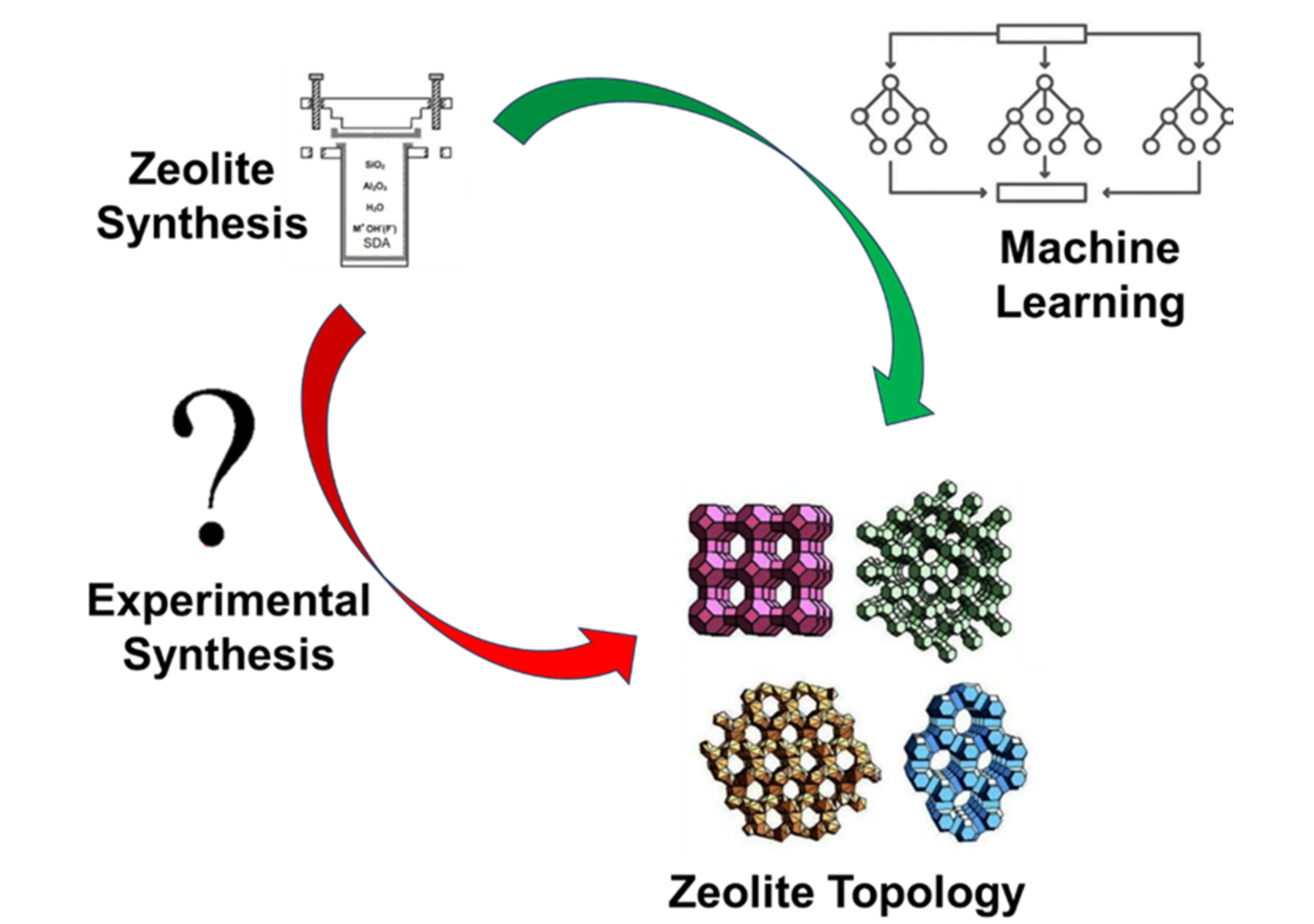
Zeolites are widely applied in various fields owing to their outstanding properties. However, our understanding on the nature of zeolite synthesis is not completed yet due to its high dimensional parameters. Machine learning has the ability to unravel fundamental relationships between complex parameters and predict the possible outcomes; thus, it can potentially reveal the nature of zeolite synthesis. This mini review highlights the current use of machine learning to comprehend the black box issue in zeolite synthesis. Conventional syntheses of zeolite were also elaborated to showcase the gap between traditional methods and machine learning approaches in zeolite synthesis. The future prospects of machine learning applications in zeolite synthesis are also discussed. This mini-review may bring crucial insights on the zeolite synthesis process.
Ahlers, C. B., Talbot, J. B., Taufiqurrahmi, N., Rahman Mohamed, A., Bhatia -, S., Arni, S., Sumari, S., Santoso, A., & Tamara, T. (2020). The Effect of Aging and Crystallization Time on the Synthesis and Characteristics of Zeolite-Y from Malang-Quartzite Silica. IOP Conference Series: Materials Science and Engineering, 833(1), 012060. https://doi.org/10.1088/1757-899X/833/1/012060
Al-Ani, A., Freitas, C., & Zholobenko, V. (2020). Nanostructured large-pore zeolite: The enhanced accessibility of active sites and its effect on the catalytic performance. Microporous and Mesoporous Materials, 293, 109805. https://doi.org/10.1016/j.micromeso.2019.109805
Al-Nahari, S., Laurencin, D., & Alonso, B. (2023). Solvent-free synthesis of zeolites: New insights into the mechanism and non-mechanochemical route. Microporous and Mesoporous Materials, 350(January), 112445. https://doi.org/10.1016/j.micromeso.2023.112445
Aragaw, T. A., & Ayalew, A. A. (2019). Removal of water hardness using zeolite synthesized from Ethiopian kaolin by hydrothermal method. Water Practice and Technology, 14(1), 145–159. https://doi.org/10.2166/wpt.2018.116
Bae, S. Y., Shin, J. S., Kim, Y. S., & Lee, J. M. (2021). Decision tree analysis on the performance of zeolite-based SCR catalysts. IFAC-PapersOnLine, 54(3), 55–60. https://doi.org/10.1016/j.ifacol.2021.08.218
Bello-Jurado, E., Schwalbe-Koda, D., Nero, M., Paris, C., Uusimäki, T., Román-Leshkov, Y., Corma, A., Willhammar, T., Gómez-Bombarelli, R., & Moliner, M. (2022). Tunable CHA/AEI Zeolite Intergrowths with A Priori Biselective Organic Structure-Directing Agents: Controlling Enrichment and Implications for Selective Catalytic Reduction of NOx. Angewandte Chemie - International Edition, 61(28), 1–6. https://doi.org/10.1002/anie.202201837
Bornes, C., Santos-Vieira, I. C. M. S., Vieira, R., Mafra, L., Simões, M. M. Q., & Rocha, J. (2023). Challenges and opportunities for zeolites in biomass upgrading: Impediments and future directions. Catalysis Today, 419(March), 114159. https://doi.org/10.1016/j.cattod.2023.114159
Butler, K. T., Davies, D. W., Cartwright, H., Isayev, O., & Walsh, A. (2018). Machine learning for molecular and materials science. Nature, 559(7715), 547–555. https://doi.org/10.1038/s41586-018-0337-2
Campanile, A., Liguori, B., Ferone, C., Caputo, D., & Aprea, P. (2022). Zeolite-based monoliths for water softening by ion exchange/precipitation process. Scientific Reports, 12(1), 1–10. https://doi.org/10.1038/s41598-022-07679-2
Chen, L., Jansson, J., Skoglundh, M., & Grönbeck, H. (2016). Mechanism for Solid-State Ion Exchange of Cu+ into Zeolites. Journal of Physical Chemistry C, 120(51), 29182–29189. https://doi.org/10.1021/acs.jpcc.6b09553
Conroy, B., Nayak, R., Hidalgo, A. L. R., & Millar, G. J. (2022). Evaluation and application of machine learning principles to Zeolite LTA synthesis. Microporous and Mesoporous Materials, 335(January), 111802. https://doi.org/10.1016/j.micromeso.2022.111802
Cundy, C. S., & Cox, P. A. (2003). The hydrothermal synthesis of zeolites: History and development from the earliest days to the present time. Chemical Reviews, 103(3), 663–701. https://doi.org/10.1021/cr020060i
Cundy, C. S., & Cox, P. A. (2005). The hydrothermal synthesis of zeolites: Precursors, intermediates and reaction mechanism. Microporous and Mesoporous Materials, 82(1–2), 1–78. https://doi.org/10.1016/j.micromeso.2005.02.016
Evans, J. D., & Coudert, F. X. (2017). Predicting the Mechanical Properties of Zeolite Frameworks by Machine Learning. Chemistry of Materials, 29(18), 7833–7839. https://doi.org/10.1021/acs.chemmater.7b02532
Fischer, M. (2020). Simulation-based evaluation of zeolite adsorbents for the removal of emerging contaminants†. Materials Advances, 1(1), 86–98. https://doi.org/10.1039/d0ma00025f
Gandhi, A., & Hasan, M. M. F. (2022). Machine learning for the design and discovery of zeolites and porous crystalline materials. Current Opinion in Chemical Engineering, 35, 100739. https://doi.org/10.1016/j.coche.2021.100739
Graça, I., Bacariza, M. C., Fernandes, A., & Chadwick, D. (2018). Desilicated NaY zeolites impregnated with magnesium as catalysts for glucose isomerisation into fructose. Applied Catalysis B: Environmental, 224, 660–670. https://doi.org/10.1016/j.apcatb.2017.11.009
Grand, J., Awala, H., & Mintova, S. (2016). Mechanism of zeolites crystal growth: New findings and open questions. CrystEngComm, 18(5), 650–664. https://doi.org/10.1039/c5ce02286j
Hanif, N., Anderson, M. W., Alfredsson, V., & Terasaki, O. (2000). The effect of stirring on the synthesis of intergrowths of zeolite Y polymorphs. Physical Chemistry Chemical Physics, 2(14), 3349–3357. https://doi.org/10.1039/B002314K
Hernando, H., Hernández-Giménez, A. M., Ochoa-Hernández, C., Bruijnincx, P. C. A., Houben, K., Baldus, M., Pizarro, P., Coronado, J. M., Fermoso, J., Čejka, J., Weckhuysen, B. M., & Serrano, D. P. (2018). Engineering the acidity and accessibility of the zeolite ZSM-5 for efficient bio-oil upgrading in catalytic pyrolysis of lignocellulose. Green Chemistry, 20(15), 3499–3511. https://doi.org/10.1039/c8gc01722k
Hewitt, D., Pope, T., Sarwar, M., Turrina, A., & Slater, B. (2022). Machine learning accelerated high-throughput screening of zeolites for the selective adsorption of xylene isomers. Chemical Science, 532, 13178–13186. https://doi.org/10.1039/d2sc03351h
Hui, K. S., & Chao, C. Y. H. (2006). Effects of step-change of synthesis temperature on synthesis of zeolite 4A from coal fly ash. Microporous and Mesoporous Materials, 88(1–3), 145–151. https://doi.org/10.1016/J.MICROMESO.2005.09.005
Jain, R., & Rimer, J. D. (2020). Seed-Assisted zeolite synthesis: The impact of seeding conditions and interzeolite transformations on crystal structure and morphology. Microporous and Mesoporous Materials, 300, 110174. https://doi.org/10.1016/J.MICROMESO.2020.110174
Jensen, Z., Kim, E., Kwon, S., Gani, T. Z. H., Román-Leshkov, Y., Moliner, M., Corma, A., & Olivetti, E. (2019). A Machine Learning Approach to Zeolite Synthesis Enabled by Automatic Literature Data Extraction. ACS Central Science, 5, 892–899. https://doi.org/10.1021/acscentsci.9b00193
Jensen, Z., Kwon, S., Schwalbe-Koda, D., Paris, C., Gómez-Bombarelli, R., Román-Leshkov, Y., Corma, A., Moliner, M., & Olivetti, E. A. (2021). Discovering Relationships between OSDAs and Zeolites through Data Mining and Generative Neural Networks. ACS Central Science, 7(5), 858–867. https://doi.org/10.1021/acscentsci.1c00024
Jia, X., Khan, W., Wu, Z., Choi, J., & Yip, A. C. K. (2019). Modern synthesis strategies for hierarchical zeolites: Bottom-up versus top-down strategies. Advanced Powder Technology, 30(3), 467–484. https://doi.org/10.1016/j.apt.2018.12.014
Jordan, M. I. & Mitchell,T. M. (2015). Machine learning: Trends,perspectives, and prospects. Science, 349(6245), 255–260.
Kadja, G. T. M., Azhari, N. J., Mardiana, S., Khalil, M., Subagjo, & Mahyuddin, M. H. (2021). Accelerated, Mesoporogen-Free Synthesis of Hierarchical Nanorod ZSM-48 Assisted by Hydroxyl Radicals. Industrial and Engineering Chemistry Research, 60(48), 17786–17791. https://doi.org/10.1021/acs.iecr.1c03586
Kadja, G. T. M., Azhari, N. J., Mukti, R. R., & Khalil, M. (2021). A Mechanistic Investigation of Sustainable Solvent-Free, Seed-Directed Synthesis of ZSM-5 Zeolites in the Absence of an Organic Structure-Directing Agent. ACS Omega, 6(1), 925–933. https://doi.org/10.1021/acsomega.0c05070
Kadja, G. T. M., Fabiani, V. A., Aziz, M. H., Fajar, A. T. N., Prasetyo, A., Suendo, V., Ng, E. P., & Mukti, R. R. (2017). The effect of structural properties of natural silica precursors in the mesoporogen-free synthesis of hierarchical ZSM-5 below 100 °C. Advanced Powder Technology, 28(2), 443–452. https://doi.org/10.1016/j.apt.2016.10.017
Kadja, G. T. M., Kadir, I. R., Fajar, A. T. N., Suendo, V., & Mukti, R. R. (2020). Revisiting the seed-assisted synthesis of zeolites without organic structure-directing agents: Insights from the CHA case. RSC Advances, 10(9), 5304–5315. https://doi.org/10.1039/c9ra10825d
Kadja, G. T. M., Mukti, R. R., Liu, Z., Rilyanti, M., Ismunandar, Marsih, I. N., Ogura, M., Wakihara, T., & Okubo, T. (2016). Mesoporogen-free synthesis of hierarchically porous ZSM-5 below 100°C. Microporous and Mesoporous Materials, 226, 344–352. https://doi.org/10.1016/j.micromeso.2016.02.007
Kadja, G. T. M., Rukmana, M. D., Mukti, R. R., Mahyuddin, M. H., Saputro, A. G., & Wungu, T. D. K. (2021). Solvent-free, small organic lactam-assisted synthesis of ZSM-5 zeolites. Materials Letters, 290, 129501. https://doi.org/10.1016/j.matlet.2021.129501
Kadja, G. T. M., Suprianti, T. R., Ilmi, M. M., Khalil, M., Mukti, R. R., & Subagjo. (2020). Sequential mechanochemical and recrystallization methods for synthesizing hierarchically porous ZSM-5 zeolites. Microporous and Mesoporous Materials, 308, 110550. https://doi.org/10.1016/j.micromeso.2020.110550
Karka, S., Kodukula, S., Nandury, S. V., & Pal, U. (2019). Polyethylenimine-Modified Zeolite 13X for CO2 Capture: Adsorption and Kinetic Studies. ACS Omega, 4(15), 16441–16449. https://doi.org/10.1021/acsomega.9b02047
Keawkumay, C., Sosa, N., Osakoo, N., Prayoonpokarach, S., Wittayakun, J., Youngjan, S., Boonyoung, P., Khemthong, P., Amedlous, A., & Mintova, S. (2025). Effect of aluminum source and water content in the precursor suspensions used for the synthesis of nanosized zeolite Y on CO₂ adsorption capacity. Microporous and Mesoporous Materials, 386, 113491. https://doi.org/10.1016/J.MICROMESO.2025.113491
Khaleque, A., Alam, M. M., Hoque, M., Mondal, S., Haider, J. Bin, Xu, B., Johir, M. A. H., Karmakar, A. K., Zhou, J. L., Ahmed, M. B., & Moni, M. A. (2020). Zeolite synthesis from low-cost materials and environmental applications: A review. Environmental Advances, 2, 100019. https://doi.org/10.1016/J.ENVADV.2020.100019
Khan, W., Jia, X., Wu, Z., Choi, J., & Yip, A. C. K. (2019). Incorporating hierarchy into conventional zeolites for catalytic biomass conversions: A review. Catalysts 9(2), 127, MDPI AG. https://doi.org/10.3390/catal9020127
Kim, N., & Min, K. (2021). Accelerated Discovery of Zeolite Structures with Superior Mechanical Properties via Active Learning. Journal of Physical Chemistry Letters, 12(9), 2334–2339. https://doi.org/10.1021/acs.jpclett.1c00339
Kumar, M., Choudhary, M. K., & Rimer, J. D. (2018). Transient modes of zeolite surface growth from 3D gel-like islands to 2D single layers. Nature Communications, 9(1), 2129. https://doi.org/10.1038/s41467-018-04296-4
Kwak, S. J., Kim, H. S., Park, N., Park, M. J., & Lee, W. B. (2021). Recent progress on Al distribution over zeolite frameworks: Linking theories and experiments. Korean Journal of Chemical Engineering, 38(6), 1117–1128. https://doi.org/10.1007/s11814-021-0796-2
Li, X., Han, H., Evangelou, N., Wichrowski, N. J., Lu, P., Xu, W., Hwang, S. J., Zhao, W., Song, C., Guo, X., Bhan, A., Kevrekidis, I. G., & Tsapatsis, M. (2023). Machine learning-assisted crystal engineering of a zeolite. Nature Communications, 14(1), 1–12. https://doi.org/10.1038/s41467-023-38738-5
Louie, S. G., Chan, Y. H., da Jornada, F. H., Li, Z., & Qiu, D. Y. (2021). Discovering and understanding materials through computation. Nature Materials, 20(6), 728–735. https://doi.org/10.1038/s41563-021-01015-1
M. I. Jordan, & T. M. Mitchell. (2015). Machine learning: Trends,perspectives, and prospects. Science, 349(6245), 255–260.
Ma, D., Li, X., Liang, J., Wang, Z., & Yang, W. (2022). Distilling seed-assisted zeolite synthesis conditions by machine learning. Microporous and Mesoporous Materials, 339(April), 112029. https://doi.org/10.1016/j.micromeso.2022.112029
Ma, S., & Liu, Z. P. (2022a). Machine learning potential era of zeolite simulation. Chemical Science, 13(18), 5055–5068. https://doi.org/10.1039/d2sc01225a
Ma, S., & Liu, Z. P. (2022b). The Role of Zeolite Framework in Zeolite Stability and Catalysis from Recent Atomic Simulation. Topics in Catalysis, 65(1–4), 59–68. https://doi.org/10.1007/s11244-021-01473-6
Ma, S., Shang, C., Wang, C. M., & Liu, Z. P. (2020). Thermodynamic rules for zeolite formation from machine learning based global optimization. Chemical Science, 11(37), 10113–10118. https://doi.org/10.1039/d0sc03918g
Mahmoodi, N. M., & Saffar-Dastgerdi, M. H. (2019). Zeolite nanoparticle as a superior adsorbent with high capacity: Synthesis, surface modification and pollutant adsorption ability from wastewater. Microchemical Journal, 145(August 2018), 74–83. https://doi.org/10.1016/j.microc.2018.10.018
Mai, H., Le, T. C., Chen, D., Winkler, D. A., & Caruso, R. A. (2022). Machine Learning in the Development of Adsorbents for Clean Energy Application and Greenhouse Gas Capture. Advanced Science, 9(36), 1–22. https://doi.org/10.1002/advs.202203899
Mardiana, S., Azhari, N. J., Ilmi, T., & Kadja, G. T. M. (2022). Hierarchical zeolite for biomass conversion to biofuel: A review. Fuel, 309(October 2021), 122119. https://doi.org/10.1016/j.fuel.2021.122119
Meftah, M., Oueslati, W., Chorfi, N., & Ben Haj Amara, A. (2017). Effect of the raw material type and the reaction time on the synthesis of halloysite based Zeolite Na-P1. Results in Physics, 7, 1475–1484. https://doi.org/10.1016/J.RINP.2017.04.013
Mguni, L. L., Ndhlovu, A., Liu, X., Hildebrandt, D., & Yao, Y. (2022). Insight into Adsorptive Desulfurization by Zeolites: A Machine Learning Exploration. Energy & Fuels, 36(8), 4427–4438. https://doi.org/10.1021/acs.energyfuels.1c03949
Mohamed, R. M., Mkhalid, I. A., & Barakat, M. A. (2015). Rice husk ash as a renewable source for the production of zeolite NaY and its characterization. Arabian Journal of Chemistry, 8(1), 48–53. https://doi.org/10.1016/j.arabjc.2012.12.013
Moliner, M., Román-Leshkov, Y., & Corma, A. (2019). Machine Learning Applied to Zeolite Synthesis: The Missing Link for Realizing High-Throughput Discovery. Accounts of Chemical Research, 52(10), 2971–2980. https://doi.org/10.1021/acs.accounts.9b00399
Muraoka, K., Sada, Y., Miyazaki, D., Chaikittisilp, W., & Okubo, T. (2019). Linking synthesis and structure descriptors from a large collection of synthetic records of zeolite materials. Nature Communications, 10(1), 1–11. https://doi.org/10.1038/s41467-019-12394-0
Murge, P., Dinda, S., & Roy, S. (2019). Zeolite-Based Sorbent for CO2 Capture: Preparation and Performance Evaluation. Langmuir. https://doi.org/10.1021/acs.langmuir.9b02259
Nikiforov, A. I., Babchuk, I. V., Vorobkalo, V. A., Chesnokov, E. A., & Chistov, D. L. (2022). Application of Machine Learning Methods to Predicting the Degree of Crystallinity of MFI Type Zeolites. Petroleum Chemistry, 62(3), 322–328. https://doi.org/10.1134/S0965544122030057
Oleksiak, M. D., Soltis, J. A., Conato, M. T., Penn, R. L., & Rimer, J. D. (2016). Nucleation of FAU and LTA Zeolites from Heterogeneous Aluminosilicate Precursors. Chemistry of Materials, 28(14), 4906–4916. https://doi.org/10.1021/acs.chemmater.6b01000
Pan, E., Kwon, S., Jensen, Z., Xie, M., Gómez-Bombarelli, R., Moliner, M., Román-Leshkov, Y., & Olivetti, E. (2024). ZeoSyn: A Comprehensive Zeolite Synthesis Dataset Enabling Machine-Learning Rationalization of Hydrothermal Parameters. ACS Central Science, 10(3), 729–743. https://doi.org/10.1021/acscentsci.3c01615
Perego, C., Bosetti, A., Ricci, M., & Millini, R. (2017). Zeolite Materials for Biomass Conversion to Biofuel. Energy and Fuels, 31(8), 7721–7733. https://doi.org/10.1021/acs.energyfuels.7b01057
Pérez-Botella, E., Palomino, M., Valencia, S., & Rey, F. (2019). Zeolites and other adsorbents. In Kaneko, K., Rodríguez-Reinoso, F. (eds) Nanoporous Materials for Gas Storage. Green Energy and Technology. Springer, SingaporeGreen Energy and Technology. https://doi.org/10.1007/978-981-13-3504-4_7
Primo, A., & Garcia, H. (2014). Zeolites as catalysts in oil refining. Chemical Society Reviews, 43(22), 7548–7561. https://doi.org/10.1039/c3cs60394f
Prodinger, S., & Derewinski, M. A. (2020). Synthetic zeolites and their characterization. In Nanoporous Materials for Molecule Separation and Conversion. INC. https://doi.org/10.1016/b978-0-12-818487-5.00003-0
Rahmah, W., Novita, T. H., Wenten, I. G., & Kadja, G. T. M. (2023). Perspective and outlook into green and effective approaches for zeolitic membrane preparation. Materials Today Sustainability, 22, 100345. https://doi.org/10.1016/j.mtsust.2023.100345
Raman, G. (2023a). Identifying extra-large pore structures in zeolites with a machine learning approach and its deployment into production. Microporous and Mesoporous Materials, 348(October 2022), 112362. https://doi.org/10.1016/j.micromeso.2022.112362
Raman, G. (2023b). Microporous and Mesoporous Materials Identifying extra-large pore structures in zeolites with a machine learning approach and its deployment into production. Microporous and Mesoporous Materials, 348(October 2022), 112362. https://doi.org/10.1016/j.micromeso.2022.112362
Salwa Mohd Nazir, L., Fong Yeong, Y., & Leng Chew, T. (2019). Effect of Alkalinity Towards the Formation of NaX Zeolite Membranes. Materials Today: Proceedings, 19, 1287–1293. https://doi.org/10.1016/J.MATPR.2019.11.135
Sasidharan, M., & Kumar, R. (1997). Effect of various inorganic cations (Li, Na, K and Cs) and silica sources on the synthesis of the silica analogue of zeolite NCL-1 (Si-NCL-1). Microporous Materials, 8(1–2), 43–47. https://doi.org/10.1016/S0927-6513(96)00056-9
Schwalbe-Koda, D., Corma, A., Román-Leshkov, Y., Moliner, M., & Gómez-Bombarelli, R. (2021). Data-Driven Design of Biselective Templates for Intergrowth Zeolites. Journal of Physical Chemistry Letters, 12(43), 10689–10694. https://doi.org/10.1021/acs.jpclett.1c03132
Schwalbe-Koda, D., Kwon, S., Paris, C., Bello-Jurado, E., Jensen, Z., Olivetti, E., Willhammar, T., Corma, A., Román-Leshkov, Y., Moliner, M., & Gómez-Bombarelli, R. (2021). A priori control of zeolite phase competition and intergrowth with high-throughput simulations. Science, 374(6565), 308–315. https://doi.org/10.1126/science.abh3350
Schwalbe-Koda, D., Santiago-Reyes, O. A., Corma, A., Román-Leshkov, Y., Moliner, M., & Gómez-Bombarelli, R. (2022). Repurposing Templates for Zeolite Synthesis from Simulations and Data Mining. Chemistry of Materials, 34(12), 5366–5376. https://doi.org/10.1021/acs.chemmater.2c00064
Shobuke, H., Matsumoto, T., Hirosawa, F., Miyagawa, M., & Takaba, H. (2022). Estimation of Adsorbed Amounts in Organoclay by Machine Learning. ACS Omega, 8(1), 1146–1153. https://doi.org/10.1021/acsomega.2c06602
Sours, T. G., & Kulkarni, A. R. (2022). Predicting Structural Properties of Pure Silica Zeolites Using Deep Neural Network Potentials. Journal of Physical Chemistry C, 127(3), 1455–1463. https://doi.org/10.1021/acs.jpcc.2c08429
Sumari, S., Fajaroh, F., Yahmin, Sholihah, N., Santoso, A., & Budianto, A. (2019). Effect of Temperature Synthesis on Structural Behaviours of NaY Zeolite Using Local Sand as A Silica Source. IOP Conference Series: Materials Science and Engineering, 515(1), 012036. https://doi.org/10.1088/1757-899X/515/1/012036
Tankersley, K. B., Dunning, N. P., Carr, C., Lentz, D. L., & Scarborough, V. L. (2020). Zeolite water purification at Tikal, an ancient Maya city in Guatemala. Scientific Reports, 10(1), 1–7. https://doi.org/10.1038/s41598-020-75023-7
Thakkar, H., Eastman, S., Hajari, A., Rownaghi, A. A., Knox, J. C., & Rezaei, F. (2016). 3D-Printed Zeolite Monoliths for CO2 Removal from Enclosed Environments. ACS Applied Materials and Interfaces, 8(41), 27753–27761. https://doi.org/10.1021/acsami.6b09647
Tomita, J., Elangovan, S. P., Itabashi, K., Chokkalingam, A., Fujinuma, H., Hao, Z., Kanno, A., Hayashi, K., Iyoki, K., Wakihara, T., & Okubo, T. (2022). OSDA-free synthesis of zeolite beta: Broadening the methodology for a successful use of the product as a seed. Advanced Powder Technology, 33(9), 103741. https://doi.org/10.1016/j.apt.2022.103741
Wang, J., Guan, Y., Zhang, Q., Zhu, H., Li, X., Li, Y., Dong, Z., Yuan, G., & Cong, Y. (2022). Well-dispersed ultrafine Pt nanoparticles anchored on oxygen-rich surface of V2CTx (MXene) for boosting hydrogen evolution reaction. Applied Surface Science, 582, 152481. https://doi.org/10.1016/J.APSUSC.2022.152481
Wang, L., Zhang, J., Yi, X., Zheng, A., Deng, F., Chen, C., Ji, Y., Liu, F., Meng, X., & Xiao, F. S. (2015). Mesoporous ZSM-5 zeolite-supported ru nanoparticles as highly efficient catalysts for upgrading phenolic biomolecules. ACS Catalysis, 5(5), 2727–2734. https://doi.org/10.1021/acscatal.5b00083
White, C. E., Provis, J. L., Proffen, T., & Van Deventer, J. S. J. (2011). Quantitative mechanistic modeling of silica solubility and precipitation during the initial period of zeolite synthesis. Journal of Physical Chemistry C, 115(20), 9879–9888. https://doi.org/10.1021/JP2006217/ASSET/IMAGES/MEDIUM/JP-2011-006217_0008.GIF
Wu, M., Zhang, S., & Ren, J. (2025). AI-empowered digital design of zeolites: Progress, challenges, and perspectives. APL Materials, 13(2), 020601. https://doi.org/10.1063/5.0253847/3333679
Wu, Q., Meng, X., Gao, X., & Xiao, F. S. (2018). Solvent-Free Synthesis of Zeolites: Mechanism and Utility [Research-article]. Accounts of Chemical Research, 51(6), 1396–1403. https://doi.org/10.1021/acs.accounts.8b00057
Xing, Q., & Blaisten-Barojas, E. (2013). A cloud computing system in windows azure platform for data analysis of crystalline materials. Concurrency and Computation: Practice and Experience, 25(15), 2157–2169. https://doi.org/https://doi.org/10.1002/cpe.2912
Xu, P., Ji, X., Li, M., & Lu, W. (2023). Small data machine learning in materials science. Npj Computational Materials, 9(1), 1–15. https://doi.org/10.1038/s41524-023-01000-z
Yang, S., Lach-hab, M., Vaisman, I. I., & Blaisten-Barojas, E. (2009). Identifying zeolite frameworks with a machine learning approach. Journal of Physical Chemistry C, 113(52), 21721–21725. https://doi.org/10.1021/jp907017u
Yang, S., Lach-Hab, M., Vaisman, I. I., & Blaisten-Barojas, E. (2008). Machine learning approach for classification of zeolite crystals. Proceedings of the 2008 International Conference on Data Mining, DMIN 2008, January, 702–706.
Yang, X., Dib, E., Lang, Q., Guo, H., Fu, G., Wang, J., Yi, Q., Zhao, H., & Valtchev, V. (2022). Silicalite-1 formation in acidic medium: Synthesis conditions and physicochemical properties. Microporous and Mesoporous Materials, 329(September 2021), 111537. https://doi.org/10.1016/j.micromeso.2021.111537
Yang, Z., Chen, B., Chen, H., & Li, H. (2023). A critical review on machine-learning-assisted screening and design of effective sorbents for carbon dioxide (CO2) capture. Frontiers in Energy Research, 10(January), 1–19. https://doi.org/10.3389/fenrg.2022.1043064
Yu, J. (2007). Chapter 3 - Synthesis of Zeolites. In J. Čejka, H. van Bekkum, A. Corma, & F. B. T.-S. in S. S. and C. Schüth (Eds.), Introduction to Zeolite Science and Practice, Studies in Surface Science and Catalysis, 168, 39–103. Elsevier. https://doi.org/10.1016/S0167-2991(07)80791-9
Zhang, C., Chu, S., Jiang, J., Zhao, J., Wen, S., Sun, B., & Xu, W. (2022). Minute-Scale Synthesis of Nano Silicalite-1 Zeolites. Frontiers in Chemistry, 10(April), 1–7. https://doi.org/10.3389/fchem.2022.860795
Zhang, H., Samsudin, I. bin, Jaenicke, S., & Chuah, G. K. (2022). Zeolites in catalysis: sustainable synthesis and its impact on properties and applications. Catalysis Science and Technology, 12(19), 6024–6039. https://doi.org/10.1039/d2cy01325h
Zhang, L., Hu, Q., Qin, Y., Liu, H., Liu, H., Cao, G., Gao, X., Song, L., & Sun, Z. (2023). Optimizing the accessibility of zeolite Y on FCC catalyst to improve heavy oil conversion capacity. Microporous and Mesoporous Materials, 359(May), 112627. https://doi.org/10.1016/j.micromeso.2023.112627
Zhang, L., Hu, Q., Qin, Y., Liu, H., Zhao, X., Gao, X., Song, L., & Sun, Z. (2022). Optimized Zeolite Distribution of FCC Catalysts for Promoting Heavy-Oil Catalytic Cracking. Industrial & Engineering Chemistry Research, 61(32), 11628–11635. https://doi.org/10.1021/acs.iecr.1c04656.
Copyright (c) 2025 Journal of Engineering and Technological Sciences

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.