Two-electron CO2 Reduction Reaction Mechanism on Nickel Cobalt Phosphate Surface Doped by Transition Metal: A DFT Study
Downloads
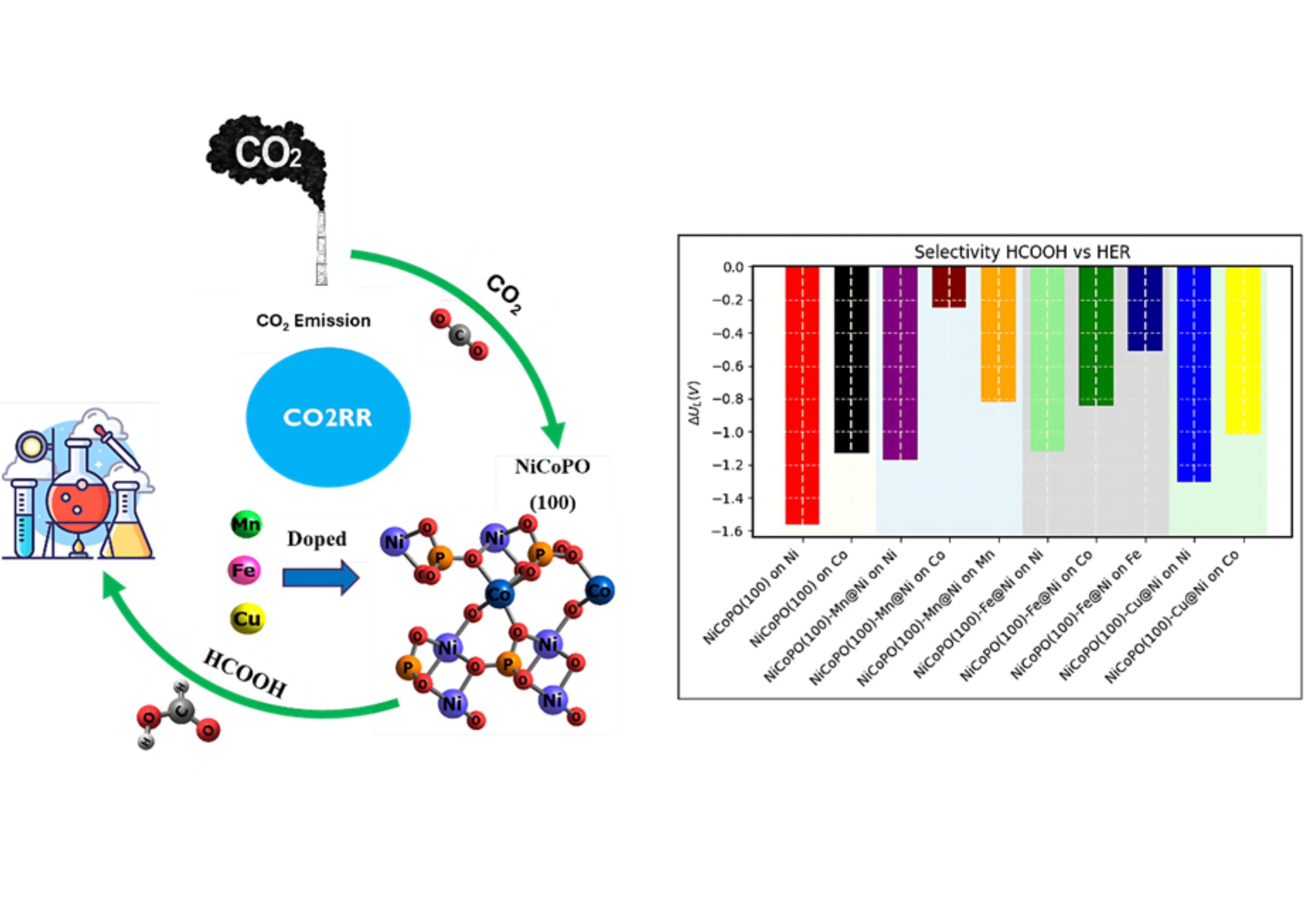
In this study, we explore the activity and selectivity of the CO2 reduction reaction (CO2RR) to CO and HCOOH on pure and transition metal-doped NiCoPO(100) surfaces using density functional theory (DFT) calculations. The novelty of this work lies in demonstrating that substitutional doping with Mn, Fe, and Cu significantly alters the thermodynamic landscape of CO₂RR, particularly in enhancing selectivity toward HCOOH. While CO remains the dominant product on most surfaces, Mn-doped NiCoPO(100) uniquely reverses this trend by reducing the limiting potential for HCOOH formation to a value lower than that for CO production. Furthermore, Mn doping suppresses the competitive hydrogen evolution reaction (HER), steering the reaction pathway more selectively toward formic acid. These findings introduce Mn-doped NiCoPO as a promising and tunable catalyst platform for selective CO₂ to HCOOH conversion, providing valuable insights for designing efficient catalysts for sustainable carbon utilization.
Alli, Y. A., Bamisaye, A., Bamidele, M. O., Etafo, N. O., Chkirida, S., Lawal, A., Hammed, V. O., Akinfenwa, A. S., Hanson, E., Nwakile, C., Kazeem, K. O., Ayanwunmi, R. J., Ige, A. S., Parga Torres, J. R., & Al Nageim, H. (2024). Transforming waste to wealth: Harnessing carbon dioxide for sustainable solutions. Results in Surfaces and Interfaces, 17, 100321. https://doi.org/10.1016/j.rsurfi.2024.100321
Alli, Y. A., Oladoye, P. O., Ejeromedoghene, O., Bankole, O. M., Alimi, O. A., Omotola, E. O., Olanrewaju, C. A., Philippot, K., Adeleye, A. S., & Ogunlaja, A. S. (2023). Nanomaterials as catalysts for CO2 transformation into value-added products: A review. Science of The Total Environment, 868, 161547. https://doi.org/10.1016/j.scitotenv.2023.161547
Bi, W., Li, X., You, R., Chen, M., Yuan, R., Huang, W., Wu, X., Chu, W. S., Wu, C., & Xie, Y. (2018). Surface Immobilization of Transition Metal Ions on Nitrogen-Doped Graphene Realizing High-Efficient and Selective CO 2 Reduction. Advanced Materials, 30, 1706617. https://doi.org/10.1002/adma.201706617
Blaylock, D. W., Ogura, T., Green, W. H., & Beran, G. J. O. (2009). Computational Investigation of Thermochemistry and Kinetics of Steam Methane Reforming on Ni(111) under Realistic Conditions. The Journal of Physical Chemistry C, 113(12), 4898–4908. https://doi.org/10.1021/jp806527q
Bolan, S., Padhye, L. P., Jasemizad, T., Govarthanan, M., Karmegam, N., Wijesekara, H., Amarasiri, D., Hou, D., Zhou, P., Biswal, B. K., Balasubramanian, R., Wang, H., Siddique, K. H. M., Rinklebe, J., Kirkham, M. B., & Bolan, N. (2024). Impacts of climate change on the fate of contaminants through extreme weather events. Science of The Total Environment, 909, 168388. https://doi.org/10.1016/j.scitotenv.2023.168388
Calle-Vallejo, F., Martínez, J. I., & Rossmeisl, J. (2011). Density functional studies of functionalized graphitic materials with late transition metals for oxygen reduction reactions. Phys. Chem. Chem. Phys., 13(34), 15639–15643. https://doi.org/10.1039/C1CP21228A
Chen, Y., Zhang, J., Tian, J., Guo, Y., Xu, F., Zhang, Y., Wang, X., Yang, L., Wu, Q., & Hu, Z. (2023). Hierarchical Ni/N/C Single-Site Catalyst Achieving Industrial-Level Current Density and Ultra-Wide Potential Plateau of High CO Faradic Efficiency for CO2 Electroreduction. Advanced Functional Materials, 33(20), 2214658. https://doi.org/10.1002/adfm.202214658
Darminto, R. P., Nuruzzahran, M. A., Syaifullah, D. A., Ilhami, H., Mobarak, N. N., AlMohamadi, H., Fathurrahman, F., Septiani, N. L. W., & Saputro, A. G. (2025). Influence of Transition Metal Doping on the Oxygen Evolution Reaction Activity of Nickel Phosphate Surface. Langmuir, 41(16), 10205–10215. https://doi.org/10.1021/acs.langmuir.4c05130
El-Gohary, A. R. M., Galal, A., & Atta, N. F. (2023). CNTs/Graphene Oxide-Nickel Phosphate Nanocomposite-Based Electrochemical Sensor for Detecting H2O2 in Human Serum. ChemistrySelect, 8(31), e202301922. https://doi.org/10.1002/slct.202301922
Ferriday, T. B., Middleton, P. H., & Kolhe, M. L. (2021). Review of the Hydrogen Evolution Reaction—A Basic Approach. Energies, 14(24). https://doi.org/10.3390/en14248535
Giannozzi, P., Baroni, S., Bonini, N., Calandra, M., Car, R., Cavazzoni, C., Ceresoli, D., Chiarotti, G. L., Cococcioni, M., Dabo, I., Corso, A. D., de Gironcoli, S., Fabris, S., Fratesi, G., Gebauer, R., Gerstmann, U., Gougoussis, C., Kokalj, A., Lazzeri, M., … Wentzcovitch, R. M. (2009). QUANTUM ESPRESSO: a modular and open-source software project for quantum simulations of materials. Journal of Physics: Condensed Matter, 21(39), 395502. https://doi.org/10.1088/0953-8984/21/39/395502
Grimme, S. (2006). Semiempirical GGA-type density functional constructed with a long-range dispersion correction. Journal of Computational Chemistry, 27(15), 1787–1799. https://doi.org/10.1002/jcc.20495
Han, M. H., Kim, D., Kim, S., Yu, S.-H., Won, D. H., Min, B. K., Chae, K. H., Lee, W. H., & Oh, H.-S. (2022). Real-Time Mimicking the Electronic Structure of N-Coordinated Ni Single Atoms: NiS-Enabled Electrochemical Reduction of CO2 to CO. Advanced Energy Materials, 12(35), 2201843. https://doi.org/10.1002/aenm.202201843
He, Q., Zhang, Y., Li, H., Yang, Y., Chen, S., Yan, W., Dong, J., Zhang, X.-M., & Fan, X. (2022). Engineering Steam Induced Surface Oxygen Vacancy onto Ni–Fe Bimetallic Nanocomposite for CO2 Electroreduction. Small, 18(15), 2108034. https://doi.org/10.1002/smll.202108034
Hou, P., Wang, X., Wang, Z., & Kang, P. (2018). Gas Phase Electrolysis of Carbon Dioxide to Carbon Monoxide Using Nickel Nitride as the Carbon Enrichment Catalyst. ACS Applied Materials & Interfaces, 10(44), 38024–38031. https://doi.org/10.1021/acsami.8b11942
Hua, W., Sun, H., Lin, L., Mu, Q., Yang, B., Su, Y., Wu, H., Lyu, F., Zhong, J., Deng, Z., & Peng, Y. (2022). A hierarchical Single-Atom Ni-N3-C catalyst for electrochemical CO2 reduction to CO with Near-Unity faradaic efficiency in a broad potential range. Chemical Engineering Journal, 446, 137296. https://doi.org/10.1016/j.cej.2022.137296
Huang, J., Mensi, M., Oveisi, E., Mantella, V., & Buonsanti, R. (2019). Structural Sensitivities in Bimetallic Catalysts for Electrochemical CO2 Reduction Revealed by Ag–Cu Nanodimers. Journal of the American Chemical Society, 141(6), 2490–2499. https://doi.org/10.1021/jacs.8b12381
Ikhwan, H., Syaifullah, D. A., Nuruzzahran, M. A., Mobarak, N. N., Wella, S. A., Fathurrahman, F., Septiani, N. L. W., & Saputro, A. G. (2025). Density functional study on the oxygen evolution reaction mechanism of transition metal doped bimetallic NiCu phosphate surface. Computational and Theoretical Chemistry, 1250, 115304. https://doi.org/10.1016/j.comptc.2025.115304
Jeong, H.-Y., Balamurugan, M., Choutipalli, V. S. K., Jeong, E., Subramanian, V., Sim, U., & Nam, K. T. (2019). Achieving highly efficient CO2 to CO electroreduction exceeding 300 mA cm−2 with single-atom nickel electrocatalysts. J. Mater. Chem. A, 7(17), 10651–10661. https://doi.org/10.1039/C9TA02405K
Kim, D., Xie, C., Becknell, N., Yu, Y., Karamad, M., Chan, K., Crumlin, E. J., Nørskov, J. K., & Yang, P. (2017). Electrochemical Activation of CO2 through Atomic Ordering Transformations of AuCu Nanoparticles. Journal of the American Chemical Society, 139(24), 8329–8336. https://doi.org/10.1021/jacs.7b03516
Kumar, T., & Eswari J, S. (2023). Review and Perspectives of Emerging Green Technology for the Sequestration of Carbon Dioxide into Value-Added Products: An Intensifying Development. Energy & Fuels, 37(5), 3570–3589. https://doi.org/10.1021/acs.energyfuels.2c04122
Li, B., Meng, T. H., Meng, X. R., & Pang, H. (2021). Nickel/Cobalt phosphate ultrathin nanosheets grown on the surface of Fe(PO3)3 nanosheets for high performance supercapacitors. Journal of Energy Storage, 42, 103082. https://doi.org/10.1016/j.est.2021.103082
Li, J., Chen, W., Lin, R., Huang, M., Wang, M., Chai, M., & Zhu, H. (2021). Thermally Evaporated Ag–Au Bimetallic Catalysts for Efficient Electrochemical CO2 Reduction. Particle & Particle Systems Characterization, 38(11), 2100148. https://doi.org/10.1002/ppsc.202100148
Li, M., Yang, K., Sun, Y., Gao, T., Nie, Z., Chen, S., Li, Q., & Duan, J. (2024). Local Steric Hindrance for CO2 Electroreduction at a Thermodynamic Potential and Wide Working Window. Advanced Energy Materials, 14(10), 2303073. https://doi.org/10.1002/aenm.202303073
Li, X., Bi, W., Chen, M., Sun, Y., Ju, H., Yan, W., Zhu, J., Wu, X., Chu, W., Wu, C., & Xie, Y. (2017). Exclusive Ni–N4 Sites Realize Near-Unity CO Selectivity for Electrochemical CO2 Reduction. Journal of the American Chemical Society, 139(42), 14889–14892. https://doi.org/10.1021/jacs.7b09074
Liu, X.-H., Jia, X.-L., Zhao, Y.-L., Zheng, R.-X., Meng, Q.-L., Liu, C.-P., Xing, W., & Xiao, M.-L. (2023). Recent advances in nickel-based catalysts for electrochemical reduction of carbon dioxide. Advanced Sensor and Energy Materials, 2(3), 100073. https://doi.org/10.1016/j.asems.2023.100073
Ma, X., Sun, F., Qin, L., Liu, Y., Kang, X., Wang, L., Jiang, D., Tang, Q., & Tang, Z. (2022). Electrochemical CO2 reduction catalyzed by atomically precise alkynyl-protected Au7Ag8{,} Ag9Cu6{,} and Au2Ag8Cu5 nanoclusters: probing the effect of multi-metal core on selectivity. Chem. Sci., 13(34), 10149–10158. https://doi.org/10.1039/D2SC02886G
Ma, Z., Wan, T., Zhang, D., Yuwono, J. A., Tsounis, C., Jiang, J., Chou, Y.-H., Lu, X., Kumar, P. V, Ng, Y. H., Chu, D., Toe, C. Y., Han, Z., & Amal, R. (2023). Atomically Dispersed Cu Catalysts on Sulfide-Derived Defective Ag Nanowires for Electrochemical CO2 Reduction. ACS Nano, 17(3), 2387–2398. https://doi.org/10.1021/acsnano.2c09473
Maulana, A. L., Putra, R. I. D., Saputro, A. G., Agusta, M. K., Nugraha, N., & Dipojono, H. K. (2019). DFT and Microkinetic Investigation of Methanol Synthesis via CO 2 Hydrogenation on Ni(111)-based Surfaces. Physical Chemistry Chemical Physics, 21(111), 20276–20286. https://doi.org/10.1039/C9CP02970B
Maulana, A. L., Saputro, A. G., Prasetyo, Y., Mahyuddin, M. H., Iqbal, M., Yudistira, H. T., Wenten, I. G., & Dipojono, H. K. (2021). Two-Electron Electrochemical Reduction of CO2 on B-Doped Ni–N–C Catalysts: A First-Principles Study. The Journal of Physical Chemistry C, 125(2), acs.jpcc.1c04986. https://doi.org/10.1021/acs.jpcc.1c04986
Moore, T. R., Damon Matthews, H., & Chavaillaz, Y. (2024). Linking Historical and Projected Trends in Extreme Precipitation with Cumulative Carbon Dioxide Emissions. Atmosphere-Ocean, 62(2), 165–182. https://doi.org/10.1080/07055900.2023.2259328
Niu, Y., Zhang, C., Wang, Y., Fang, D., Zhang, L., & Wang, C. (2021). Confining Chainmail-Bearing Ni Nanoparticles in N-doped Carbon Nanotubes for Robust and Efficient Electroreduction of CO2. ChemSusChem, 14(4), 1140–1154. https://doi.org/10.1002/cssc.202002596
Norskov, J., Rossmeisl, J., Logadottir, A., Lindqvist, L., Kitchin, J. R., Bligaard, T., & Jonsson, H. (2004). Origin of the overpotential for oxygen reduction at a fuel-cell cathode. The Journal of Physical Chemistry B, 108(46), 17886–17892. https://doi.org/10.1021/jp047349j
Nuruddin, A., Saputro, A. G., Maulana, A. L., Fajrial, A. K., Shukri, G., Mahyuddin, M. H., Aprilyanti, F. D., Harimawan, A., & Dipojono, H. K. (2023). Enhancing oxygen reduction reaction activity of pyrolyzed Fe–N–C catalyst by the inclusion of BN dopant at the graphitic edges. Applied Surface Science, 608, 155203. https://doi.org/10.1016/j.apsusc.2022.155203
Nuruddin, A., Saputro, A. G., Maulana, A. L., Rusydi, F., Akbar, F. T., Yudistira, H. T., & Dipojono, H. K. (2024). Selectivity of CO2 reduction reaction to CO on the graphitic edge active sites of Fe-single-atom and dual-atom catalysts: A combined DFT and microkinetic modeling. Carbon Resources Conversion, 7(1), 100185. https://doi.org/10.1016/j.crcon.2023.05.004
Nuruzzahran, M. A., Syaifullah, D. A., Mariani, C. C., Fathurrahman, F., Yuliarto, B., Dipojono, H. K., AlMohamadi, H., Wella, S. A., Wulan Septiani, N. L., & Saputro, A. G. (2025). Oxygen evolution reaction activity of Ni3(PO4)2 and bimetallic Ni3M3(PO4)4 (M = Mn, Fe, Co): Insights from DFT and experimental validation. Fuel, 385, 134183. https://doi.org/10.1016/j.fuel.2024.134183
Perdew, J. P., Burke, K., & Ernzerhof, M. (1996). Generalized Gradient Approximation Made Simple. Phys. Rev. Lett., 77(18), 3865–3868. https://doi.org/10.1103/PhysRevLett.77.3865
Peterson, A. A., Abild-Pedersen, F., Studt, F., Rossmeisl, J., & Nørskov, J. K. (2010). How copper catalyzes the electroreduction of carbon dioxide into hydrocarbon fuels. Energy Environ. Sci., 3(9), 1311–1315. https://doi.org/10.1039/C0EE00071J
Qian, L., & Miao, Y. (2019). Nanosheet organized flower-like Co/Zn phosphate on nickel foam for efficient water splitting in both acid and basic solutions. Polyhedron, 160, 213–218. https://doi.org/10.1016/j.poly.2018.12.050
Saputro, A. G., Maulana, A. L., Aprilyanti, F. D., & Dipojono, H. K. (2021a). Theoretical Study of Direct Carbon Dioxide Conversion to Formic Acid on Transition Metal-doped Subnanometer Palladium Clusters. Journal of Engineering and Technological Sciences, 53(4), 210402. https://doi.org/10.5614/j.eng.technol.sci.2021.53.4.2
Saputro, A. G., Maulana, A. L., Fathurrahman, F., Shukri, G., Mahyuddin, M. H., Agusta, M. K., Wungu, T. D. K., & Dipojono, H. K. (2021b). Density functional and microkinetic study of CO2 hydrogenation to methanol on subnanometer Pd cluster doped by transition metal (M= Cu, Ni, Pt, Rh). International Journal of Hydrogen Energy, 46(27), 14418–14428. https://doi.org/10.1016/j.ijhydene.2021.02.009
Saxena, A., Liyanage, W. P. R., Kapila, S., & Nath, M. (2022). Nickel selenide as an efficient electrocatalyst for selective reduction of carbon dioxide to carbon-rich products††Electronic supplementary information (ESI) available: GC-TCD spectra, product quantification details from NMR spectra, calculation of farada. Catalysis Science & Technology, 12(15), 4727–4739. https://doi.org/10.1039/d2cy00583b
Septiani, N. L. W., Kaneti, Y. V., Fathoni, K. B., Guo, Y., Ide, Y., Yuliarto, B., Jiang, X., Nugraha, Dipojono, H. K., Golberg, D., & Yamauchi, Y. (2020a). Tailorable nanoarchitecturing of bimetallic nickel–cobalt hydrogen phosphate via the self-weaving of nanotubes for efficient oxygen evolution. J. Mater. Chem. A, 8(6), 3035–3047. https://doi.org/10.1039/C9TA13442E
Septiani, N. L. W., Kaneti, Y. V., Fathoni, K. B., Kani, K., Allah, A. E., Yuliarto, B., Nugraha, Dipojono, H. K., Alothman, Z. A., Golberg, D., & Yamauchi, Y. (2020b). Self-Assembly of Two-Dimensional Bimetallic Nickel–Cobalt Phosphate Nanoplates into One-Dimensional Porous Chainlike Architecture for Efficient Oxygen Evolution Reaction. Chemistry of Materials, 32(16), 7005–7018. https://doi.org/10.1021/acs.chemmater.0c02385
Shaikh, A. R., Vidal-López, A., Brotons-Rufes, A., Pajski, J. J., Zafar, S., Mahmood, R. A., Khan, M. U., Poater, A., Chawla, M., & Cavallo, L. (2024). Amino acid ionic liquids as efficient catalysts for CO2 capture: A combined static and dynamic approach. Results in Surfaces and Interfaces, 14, 100175. https://doi.org/10.1016/j.rsurfi.2023.100175
Syaifullah, D. A., Nuruzzahran, M. A., Idhola, R. D., Mobarak, N. N., AlMohamadi, H., Patole, S., Wella, S. A., Akbar, F. T., Rizkiana, J., Wulan Septiani, N. L., & Saputro, A. G. (2026). Tuning oxygen evolution activity via transition metal doping in bimetallic nickel phosphates. Applied Surface Science, 717, 164846. https://doi.org/10.1016/j.apsusc.2025.164846
Tan, D., Cui, C., Shi, J., Luo, Z., Zhang, B., Tan, X., Han, B., Zheng, L., Zhang, J., & Zhang, J. (2019). Nitrogen-carbon layer coated nickel nanoparticles for efficient electrocatalytic reduction of carbon dioxide. Nano Research, 12(5), 1167–1172. https://doi.org/10.1007/s12274-019-2372-1
Tripkovic, V., Vanin, M., Karamad, M., Björketun, M. E., Jacobsen, K. W., Thygesen, K. S., & Rossmeisl, J. (2013). Electrochemical CO2 and CO Reduction on Metal-Functionalized Porphyrin-like Graphene. The Journal of Physical Chemistry C, 117(18), 9187–9195. https://doi.org/10.1021/jp306172k
Wang, D., Wang, Y., Fu, Z., Xu, Y., Yang, L.-X., Wang, F., Guo, X., Sun, W., & Yang, Z.-L. (2021). Cobalt–Nickel Phosphate Composites for the All-Phosphate Asymmetric Supercapacitor and Oxygen Evolution Reaction. ACS Applied Materials & Interfaces, 13(29), 34507–34517. https://doi.org/10.1021/acsami.1c04614
Wijayanti, D. P., Nuruzzahran, M. A., Syaifullah, D. A., Adhika, D. R., AlMohamadi, H., Mobarak, N. N., Septiani, N. L. W., & Saputro, A. G. (2025). Hydrogen evolution reaction mechanism on pristine and defective nickel phosphate surfaces. Results in Surfaces and Interfaces, 18, 100431. https://doi.org/10.1016/j.rsurfi.2025.100431
Xu, C., Vasileff, A., Jin, B., Wang, D., Xu, H., Zheng, Y., & Qiao, S.-Z. (2020). Graphene-encapsulated nickel–copper bimetallic nanoparticle catalysts for electrochemical reduction of CO2 to CO. Chem. Commun., 56(76), 11275–11278. https://doi.org/10.1039/D0CC04779A
Yang, K., Li, M., Gao, T., Xu, G., Li, D., Zheng, Y., Li, Q., & Duan, J. (2024). An acid-tolerant metal-organic framework for industrial CO2 electrolysis using a proton exchange membrane. Nature Communications , 15(1). https://doi.org/10.1038/s41467-024-51475-7
Yusuf, N., Almomani, F., & Qiblawey, H. (2023). Catalytic CO2 conversion to C1 value-added products: Review on latest catalytic and process developments. Fuel, 345, 128178. https://doi.org/10.1016/j.fuel.2023.128178
Zhang, X., Shang, N., Gao, S., Wang, C., Gao, Y., & Wang, Z. (2019). Surfactant assisted self-assembly of NiCo phosphate with superior electrochemical performance for supercapacitor. Applied Surface Science, 483, 529–535. https://doi.org/10.1016/j.apsusc.2019.03.339
Zhang, Y., He, Z., Dong, Q., Tang, X., Yang, L., Huang, K., Zou, Z., Jiang, X., & Xiong, X. (2022). 3D CoxP@NiCo-LDH heteronanosheet array: As a high sensitivity sensor for glucose. Microchemical Journal, 172, 106923. https://doi.org/10.1016/j.microc.2021.106923
Zhao, H., & Yuan, Z.-Y. (2020). Insights into Transition Metal Phosphate Materials for Efficient Electrocatalysis. ChemCatChem, 12(15), 3797–3810. https://doi.org/10.1002/cctc.202000360
Zhao, R., Wang, Y., Ji, G., Zhong, J., Zhang, F., Chen, M., Tong, S., Wang, P., Wu, Z., Han, B., & Liu, Z. (2023). Partially Nitrided Ni Nanoclusters Achieve Energy-Efficient Electrocatalytic CO2 Reduction to CO at Ultralow Overpotential. Advanced Materials, 35(5), 2205262. https://doi.org/10.1002/adma.202205262
Zhao, S., Xiao, N., Li, H., Guo, Z., Bai, J., Xiao, J., Guo, H., Ma, X., & Qiu, J. (2021). A nickel-nitrogen-doped carbon foam as monolithic electrode for highly efficient CO2 electroreduction. Journal of CO2 Utilization, 49, 101549. https://doi.org/10.1016/j.jcou.2021.101549
Zheng, T., Jiang, K., Ta, N., Hu, Y., Zeng, J., Liu, J., & Wang, H. (2019). Large-Scale and Highly Selective CO2 Electrocatalytic Reduction on Nickel Single-Atom Catalyst. Joule, 3(1), 265–278. https://doi.org/10.1016/j.joule.2018.10.015
Zhou, C., Gao, T., Wang, Y., Liu, Q., & Xiao, D. (2019). Through a hydrothermal phosphatization method synthesized NiCo and Fe-based electrodes for high-performance battery-supercapacitor hybrid device. Applied Surface Science, 475, 729–739. https://doi.org/10.1016/j.apsusc.2018.12.299
Copyright (c) 2026 Journal of Engineering and Technological Sciences

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.